Cancer Metabolic Imaging & Therapy Lab | Viswanath Lab
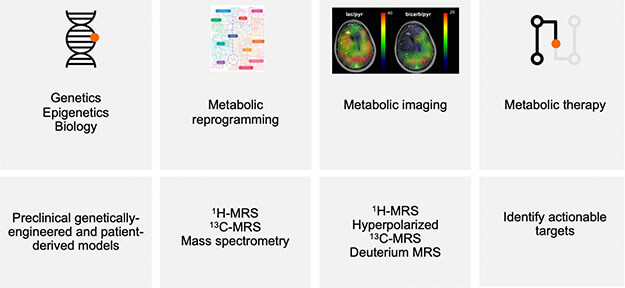
Our research program is driven by a bold vision: to uncover how cancer-triggering events reshape the metabolic landscape of both tumors and the immune system and use that knowledge to develop groundbreaking therapies and imaging tools for brain cancer. Through a deeply interdisciplinary approach, we fuse our strengths in metabolomics, immunology, drug discovery, and advanced imaging to craft an integrated strategy that can revolutionize how brain tumors are diagnosed and treated.
Collaboration is at the core of what we do. Our team closely partners with clinical experts at UCSF and other leading institutions, enabling us to rapidly translate discoveries from bench to bedside. Together, we aim to redefine how brain tumors are diagnosed and treated through precision-guided metabolic therapies and imaging.
Research
Advancing Brain Tumor Research with Hyperpolarized 13C Imaging
Our laboratory is at the forefront of developing cutting-edge metabolic imaging tools to illuminate how cancer alters metabolic processes in the brain. With a deep focus on hyperpolarized 13C magnetic resonance spectroscopy (MRS), we are pioneering techniques that allow us to visualize oncogenic events with unprecedented clarity.
Among our most exciting innovations are hyperpolarized 13C agents- such as alanine, glucose, and gluconolactone- that reveal telomere maintenance mechanisms in brain tumors. Today, we’re expanding our efforts to tackle meningiomas, developing tailored metabolic imaging biomarkers that reflect their molecular profiles. By turning invisible processes into actionable insights, our research is transforming how brain tumors are understood, diagnosed, and ultimately treated.
Relevant publications:
- Viswanath P, Batsios G, Mukherjee J, Gillespie AM, Larson PEZ, Luchman HA, Phillips JJ, Costello JF, Pieper RO, and Ronen SM. Non-invasive assessment of telomere maintenance mechanisms in brain tumors. Nature Communications. 2021; 12(1):92. doi: 10.1038/s41467-020-20312-y. PMID: 33397920.
- Viswanath P, Batsios G, Ayyappan V, Taglang C, Gillespie AM, Larson PEZ, Luchman HA, Costello JF, Pieper RO, Ronen SM. Metabolic imaging detects elevated glucose flux through the pentose phosphate pathway associated with TERT expression in low-grade gliomas. Neuro-oncology 2021; 23(9):1509-1522. doi: 10.1093/neuonc/noab093. PMID: 33864084.
Charting New Frontiers with Deuterium Metabolic Imaging
In our lab, we are transforming how brain tumors are visualized and understood- one molecule at a time. Using deuterium metabolic imaging, we have pioneered the use of deuterated glucose and pyruvate to reveal tumor burden and track therapeutic response in both adult and pediatric brain cancers with IDH and oncohistone mutations.
Now, we are taking the next leap by developing deuterated alpha-ketoglutarate as a highly specific tracer for detecting IDH mutations in gliomas. These breakthroughs hold the promise of precision imaging, paving the way for smarter diagnostics, real-time treatment monitoring, and more targeted care for brain tumor patients.
Relevant publications:
- Taglang C, Batsios G, Mukherjee J, Tran M, Gillespie AM, Hong D, Ronen SM, Luchman HA, Pieper RO, Viswanath P. Deuterium magnetic resonance spectroscopy enables non-invasive metabolic imaging of tumor burden and response to therapy in low-grade gliomas. Neuro-Oncology, 2022; 24(7): 1101-1112. doi: 10.1093/neuonc/noac022. PMID: 35091751; PMCID: PMC9248401.
- Batsios G, Taglang T, Tran M, Stevers N, Barger C, Gillespie AM, Ronen SM, Costello JF, Viswanath P. Deuterium metabolic imaging reports on TERT expression and early response to therapy in cancer. Clinical Cancer Research, 2022; 28(16):3526-3536. doi: 10.1158/1078-0432.CCR-21-4418. PMID: 35679032; PMCID: PMC9378519.
- Batsios G, Taglang C, Udutha S, Gillespie AM, Phoenix T, Mueller S, Venneti S, Koschmann C, Viswanath P. Lactylation fuels nucleotide biosynthesis and facilitates deuterium metabolic imaging of tumor proliferation in H3K27M-mutant gliomas. bioRxiv, 2025, doi: 10.1101/2025.01.02.631150. PMID: 39803472
Targeting Metabolic Vulnerabilities in Brain Tumors
- Udutha S, Taglang C, Batsios G, Gillespie AM, Tran M, Hoeve JT, Graeber TG, Viswanath P. Combined inhibition of de novo glutathione and nucleotide biosynthesis is synthetically lethal in glioblastoma. Cell Reports, 2025, 44(5):115596. doi: 10.1016/j.celrep.2025.115596. PMID: 40253695.
- Udutha S, Batsios G, Taglang C, Gillespie AM, Viswanath P. The 1p/19q co-deletion induces targetable and imageable vulnerabilities in glucose metabolism in oligodendrogliomas. biorxiv, 2025, doi: 10.1101/2025.05.20.655097. PMID: 40475477.
Exploring Immunometabolism in the Tumor Microenvironment
An exciting new frontier of research in our laboratory is focused on unraveling the metabolic dynamics of the tumor microenvironment, where cancer cells and immune cells collide in a biochemical tug-of-war. Our goal is to gain a mechanistic understanding of how tumors reprogram metabolic pathways to suppress immune responses and promote their own survival.
Our team is developing powerful metabolic imaging biomarkers that illuminate the immunometabolic landscape of both adult and pediatric brain tumors. These tools allow us not only to visualize metabolic changes in real time but also to pinpoint emerging therapeutic targets that arise from disrupted immunometabolism. By bridging metabolism, immunology, and imaging, we hope to reimagine cancer diagnosis and therapy- from identifying hidden metabolic choke points to building smarter therapies that restore the balance in favor of the immune system.
News
American Cancer Society Grant Awarded December 2024
Dr. Viswanath's research identifying and inhibiting metabolic pathways in glioblastoma macrophages is supported by the American Cancer Society's Discovery Boost Grant.
ABTA Grant, November 2024
The American Brain Tumor Association Research Collaboration Grants are two-year, $200,000 grants for multi-investigator and multi-institutional brain tumor collaborative research projects.
Game-Changer Grant - ChadTough Defeat DIPG Foundation, July 2024
Pavithra Viswanath, PhD, received a Game-Changer Grant from ChadTough Defeat DIPG Foundation, along with its Family Partners and Research Partners. These grants support research projects that represent an innovative approach to a major challenge in the study of DIPG/DMG, particularly aggressive and difficult-to-treat brain tumors, typically found in children.
Spotlight: Delivering Science that Makes a Difference, April 2024
“I love the science we are doing. I believe it has the potential to make a difference for the patient,” said researcher Pavithra Viswanath, PhD, principal investigator of the Cancer Metabolic Imaging and Therapy Lab. “At UCSF, we have a fantastic setup to develop novel therapies, and there’s no place better than Radiology to develop metabolic imaging biomarkers.”
Read more: How Viswanath Uses Metabolism to Understand Brain Tumors
Opportunities
Join our team at UCSF: Postdoctoral Scholar in Brain Tumor Research!
Highly motivated researchers with a passion for impacting brain tumor treatment are welcome to join the dynamic team at the University of California, San Francisco (UCSF), a world-renowned institution leading cutting-edge translational research.
About the opportunity
This Postdoctoral Scholar position offers an opportunity to contribute directly to clinical advancements by investigating brain tumor metabolism, therapy, and imaging. The research bridges the gap between basic science and clinical application, aiming to improve patient outcomes through innovative approaches.
Key Qualifications
- PhD or MD/PhD in Biomedical Sciences.
- Strong background in cancer metabolism, immunology, biochemistry, and/or imaging.
- Passion for brain tumor research and collaborative spirit.
- Excellent communication skills and the ability to work independently and collaboratively.
Research Focus
The laboratory integrates multiple disciplines, including metabolomics, immunology, drug discovery, and advanced imaging, to develop an integrated metabolic therapy and imaging strategy for brain tumors.
Location
University of California, San Francisco (UCSF), located in the vibrant heart of the Bay Area, is a world-renowned research institution with a strong emphasis on collaboration and innovation in brain tumor research.
Apply Now
Interested candidates should send a CV, cover letter, and contact information for three references to:
Dr. Pavithra Viswanath
Email: [email protected]
Publications
Featured Publications
- Udutha S, Batsios G, Taglang C, Gillespie AM, Viswanath P. The 1p/19q co-deletion induces targetable and imageable vulnerabilities in glucose metabolism in oligodendrogliomas. bioRxiv, 2025. doi: 10.1101/2025.05.20.655097. PMID: 40475477; PMCID: PMC12139843.
- Udutha S, Taglang C, Batsios G, Gillespie AM, Tran M, Hoeve JT, Graeber TG, Viswanath P. Combined inhibition of de novo glutathione and nucleotide biosynthesis is synthetically lethal in glioblastoma. Cell Rep, 2025, 44(5): 115596. doi: 10.1016/j.celrep.2025.115596. PMID: 40253695; PMCID: PMC12204606.
- Batsios G, Taglang C, Udutha S, Gillespie AM, Robinson SP, Phoenix T, Mueller S, Venneti S, Koschmann C, Viswanath P. Lactylation fuels nucleotide biosynthesis and facilitates deuterium metabolic imaging of tumor proliferation in H3K27M-mutant gliomas. bioRxiv, 2025. doi: 10.1101/2025.01.02.631150. PMID: 39803472; PMCID: PMC11722398.
- Batsios G, Udutha S, Taglang C, Gillespie AM, Lau B, Ji S, Phoenix T, Mueller S, Venneti S, Koschmann C, Viswanath P. GABA production induced by imipridones is a targetable and imageable metabolic alteration in diffuse midline gliomas. bioRxiv, 2024. doi: 10.1101/2024.06.07.597982. PMID: 38915617; PMCID: PMC11195108.
- Batsios G, Taglang C, Gillespie AM, Viswanath P. Imaging telomerase reverse transcriptase expression in oligodendrogliomas using hyperpolarized d-[1-13C]-gluconolactone. Neurooncol Adv, 2023, 5(1): vdad092. doi: 10.1093/noajnl/vdad092. PMID: 37600229; PMCID: PMC10433788.
- Batsios G, Taglang C, Tran M, Stevers N, Barger C, Gillespie AM, Ronen SM, Costello JF, Viswanath P. Deuterium Metabolic Imaging Reports on TERT Expression and Early Response to Therapy in Cancer. Clin Cancer Res, 2022, 28(16): 3526-3536. doi: 10.1158/1078-0432.CCR-21-4418. PMID: 35679032; PMCID: PMC9378519.
- Taglang C, Batsios G, Mukherjee J, Tran M, Gillespie AM, Hong D, Ronen SM, Luchman HA, Pieper RO, Viswanath P. Deuterium magnetic resonance spectroscopy enables noninvasive metabolic imaging of tumor burden and response to therapy in low-grade gliomas. Neuro Oncol, 2022, 24(7): 1101-1112. doi: 10.1093/neuonc/noac022. PMID: 35091751; PMCID: PMC9248401.
- Batsios G, Taglang C, Cao P, Gillespie AM, Najac C, Subramani E, Wilson DM, Flavell RR, Larson PEZ, Ronen SM, Viswanath P. Imaging 6-Phosphogluconolactonase Activity in Brain Tumors in vivo using hyperpolarized d-[1-13C]-gluconolactone. Front Oncol, 2021, 11: 589570. doi: 10.3389/fonc.2021.589570. PMID: 33937017; PMCID: PMC8082394.
- Viswanath P, Batsios G, Ayyappan V, Taglang C, Gillespie AM, Larson PEZ, Luchman HA, Costello JF, Pieper RO, Ronen SM. Metabolic imaging detects elevated glucose flux through the pentose phosphate pathway associated with TERT expression in low-grade gliomas. Neuro Oncol, 2021, 23(9): 1509-1522. doi: 10.1093/neuonc/noab093. PMID: 33864084; PMCID: PMC8408874.
- Viswanath P, Batsios G, Mukherjee J, Gillespie AM, Larson PEZ, Luchman HA, Phillips JJ, Costello JF, Pieper RO, Ronen SM. Non-invasive assessment of telomere maintenance mechanisms in brain tumors. Nat Commun, 2021, 12(1): 92. doi: 10.1038/s41467-020-20312-y. PMID: 33397920; PMCID: PMC7782549.
- Viswanath P, Radoul M, Izquierdo-Garcia JL, Luchman HA, Cairncross JG, Pieper RO, Phillips JJ, Ronen SM. Mutant IDH1 gliomas downregulate phosphocholine and phosphoethanolamine synthesis in a 2-hydroxyglutarate- dependent manner. Cancer Metab, 2018, 6: 3. doi: 10.1186/s40170-018-0178-3. PMID: 29619216; PMCID: PMC5881177.
- Viswanath P, Radoul M, Izquierdo-Garcia JL, Ong WQ, Luchman HA, Cairncross JG, Huang B, Pieper RO, Phillips JJ, Ronen SM. 2-Hydroxyglutarate-Mediated Autophagy of the Endoplasmic Reticulum Leads to an Unusual Downregulation of Phospholipid Biosynthesis in Mutant IDH1 Gliomas. Cancer Res, 2018, 1;78(9): 2290-2304. doi: 10.1158/0008-5472.CAN-17-2926. Epub 2018 Jan 22. PMID: 29358170; PMCID: PMC5932252.
- Viswanath P, Najac C, Izquierdo-Garcia JL, Pankov A, Hong C, Eriksson P, Costello JF, Pieper RO, Ronen SM. Mutant IDH1 expression is associated with down-regulation of monocarboxylate transporters. Oncotarget, 2016, 7(23): 34942-55. doi: 10.18632/oncotarget.9006. PMID: 27144334; PMCID: PMC5085201.
- Izquierdo-Garcia JL, Viswanath P, Eriksson P, Cai L, Radoul M, Chaumeil MM, Blough M, Luchman HA, Weiss S, Cairncross JG, Phillips JJ, Pieper RO, Ronen SM. IDH1 Mutation Induces Reprogramming of Pyruvate Metabolism. Cancer Res, 2015, 75(15): 2999-3009. doi: 10.1158/0008-5472.CAN-15-0840. Epub 2015 Jun 4. PMID: 26045167; PMCID: PMC4526330.
For a complete list of publications, visit Dr. Pavithra Viswanath's UCSF Profiles page.
People
 Pavithra Viswanath, PhD - Principal Investigator
Pavithra Viswanath, PhD - Principal Investigator
Dr. Viswanath is an Associate Professor in Residence in the Department of Radiology and Biomedical Imaging at UCSF. With a Ph.D. in Biochemistry from the Indian Institute of Science, Bangalore, she brings a multidisciplinary approach to biomedical research- blending biochemistry, immunology, drug discovery, and imaging to illuminate new pathways in cancer diagnosis and treatment.
Her work focuses on decoding cancer’s metabolic fingerprints to develop innovative imaging biomarkers and therapeutic strategies. By bridging basic science with clinical application, her work aims to transform how tumors are diagnosed, classified, and treated. At UCSF, Dr. Viswanath leads a close-knit team of biomedical engineers, chemists, and biologists, each bringing a unique lens to the shared mission of advancing cancer care. Together, they investigate how metabolic reprogramming in tumors can be exploited for precision imaging and therapy, striving to push the boundaries of what’s possible in cancer care.
Outside the lab, Dr. Viswanath enjoys reading, hiking, yoga, and playing board games with her husband and children.
 Anne Marie Gillespie, Lab Manager
Anne Marie Gillespie, Lab Manager
Anne Marie graduated with an MSc from NUIG Ireland. Anne Marie has previously worked with Dr. Charles Epstein and Dr. Sam Hawgood at UCSF.
 Georgios Batsios, PhD, Specialist
Georgios Batsios, PhD, Specialist
Georgios is a transplant from a small country town in Northern Greece. After acquiring his diploma in Electrical and Computer Engineering from National Technical University of Athens, Greece, he moved to Switzerland to advance his studies. There he worked as a researcher in ETH Zurich pursuing his PhD in Biomedical Engineering with a focus on MR Imaging. His research in the Viswanath lab focuses on advancing preclinical metabolic imaging of cancer. Specifically, he is developing methods to investigate the metabolism of brain tumor models using 1H, 2H and 13C MR imaging and spectroscopic techniques. Outside the lab he enjoys baking, hiking, strolling the farmer's market, long zoom chats with his family and exploring the beauty of nature with his family.

Kirti Singh, Postdoctoral Scholar
Kirti Singh earned her PhD in molecular pharmacology from Dr. Moniri’s lab at Mercer University, Atlanta, Georgia, USA. After graduating she worked at Eli Lilly as a postdoctoral scholar in early phase preclinical drug development for novel target identification and characterization in pulmonary fibrosis. She joined Viswanath lab in November 2024 as a postdoc, with the focus to investigate brain tumor metabolism in preclinical models to advance metabolic imaging and therapy. Apart from lab, she enjoys cooking, hiking, and exploring national parks.
 Celine Taglang, PhD, Specialist
Celine Taglang, PhD, Specialist
Celine obtained her PhD in Chemistry at Paris Saclay University and the French Atomic Energy Commission (CEA). She developed an enantiospecific C-H activation reaction followed by deuterium incorporation at stereogenic centers, using ruthenium nanoparticles and hydrogen gas. Celine joined UCSF in 2016 to use her experience in medicinal chemistry and radiochemistry to improve 13C labeling and hyperpolarization of biocompatible agents for MRI with applications from enzymatic activity to animal models. Since August 2020, her research in the Viswanath Lab has focused on deuterium magnetic resonance spectroscopic imaging with intravenous infusion of nonradioactive 2H-labeled substrates to better reveal metabolism in the brain of animal models and differences between normal brain and tumor tissue. She also likes yoga and challenged herself in 2018 for the San Francisco Marathon. Next step? The NYC marathon!
 Nathaniel "Nate" Goodwin, Staff Research Associate
Nathaniel "Nate" Goodwin, Staff Research Associate
Nate grew up in Charleston, West Virginia and El Paso, Texas prior to attending UC Berkeley where he earned a BS in Chemistry and a BA in Mathematics and Molecular Cellular Biology. After graduating, Nate worked as a high school chemistry teacher in Oakland until 2024 when he joined the Viswanath Lab at UCSF. He now studies metabolism in meningiomas with applications in clinical imaging, targeted therapies, and assessing therapeutic response. Additionally, he is interested in materials science and tunable nanomaterials for targeted therapy. Outside of the lab, Nate loves to pick up new hobbies including biking and fixing up bikes, playing board games, watching reality competition shows, and learning new crafts like crotchet and quilting.
 Suresh Udutha, PhD, Postdoctoral Scholar
Suresh Udutha, PhD, Postdoctoral Scholar
Dr. Suresh Udutha is a Postdoctoral Scholar in the Department of Radiology and Biomedical Imaging at UCSF. He earned his Ph.D. in Chemistry with a specialization in Mass Spectrometry from CSIR–Indian Institute of Chemical Technology in India. His research focuses on investigating metabolic alterations in glioblastoma models and exploring the underlying metabolic pathways involved in disease progression and therapeutic response. He is particularly interested in identifying metabolic biomarkers during combination drug therapies, with the goal of advancing precision medicine in cancer treatment. His work integrates mass spectrometry-based metabolomics to characterize tumor biology and uncover metabolic vulnerabilities that can be targeted therapeutically.
Principal Investigator
- Research Directory
- Abdominal and Pelvic MRI
- Arthritis Imaging Lab (Li Lab)
- Baby Brain Research Group
- Biomagnetic Imaging Laboratory
- Biomechanics & Musculoskeletal Imaging Lab
- Bone Quality Research Lab
- BrainChange Study
- Breast Imaging Research Group
- Breast and Bone Density Group
- Cardiothoracic Imaging Imaging Research
- Center for Molecular and Functional Imaging (CMFI)
- Contrast Material and CT Translational Research Lab
- Evans Lab
- Focused Ultrasound Lab
- High Field MRI Center
- Imaging Research for Neurodevelopment
- Interventional Radiology Research Lab
- Larson Group
- Lupo Lab
- Multimodal Metabolic Brain Imaging
- Musculoskeletal Quantitative Imaging Research
- Neural Connectivity Lab
- Osteoid Osteomas HIFU Clinical Trial
- PET/SPECT Radiochemistry
- PSMA PET Scan
- Physics Research Laboratory
- Program for Molecular Imaging and Targeted Therapy
- Prostate Cancer Imaging Lab (Kurhanewicz)
- Quantitative Biomarkers & AI in MSK Imaging
- Research
- Sarah J. Nelson Lab
- Surbeck Laboratory
- Translational Metabolic Imaging Lab
- Vascular Imaging Research Center
- Viswanath Lab
- Wilson Lab
- Imaging Research Symposium
- Research Conference
- UCSF Radiology at RSNA
- Core Services
Contact
Byers Hall 1700 4th Street,
Room 304, Box 2532
San Francisco, CA 94158-2330

