Vascular Imaging Research Center

You name a vessel, we'll image it! We apply advanced cardiovascular imaging techniques to a range of diseases with the practical goal of improving clinical management. See "Projects" below for details about our research.
Research Projects
Abdominal Aortic Aneurysms
- 1R01HL123759-01A1 "Hemodynamic and inflammatory imaging in evaluation of abdominal aortic aneurysms" (Hope, 2015)
- 1R01HL114118-01A1 "MRI of Structure and Function in Assessing Hemodynamic Impact on AAA Evolution" (Saloner, 2014)
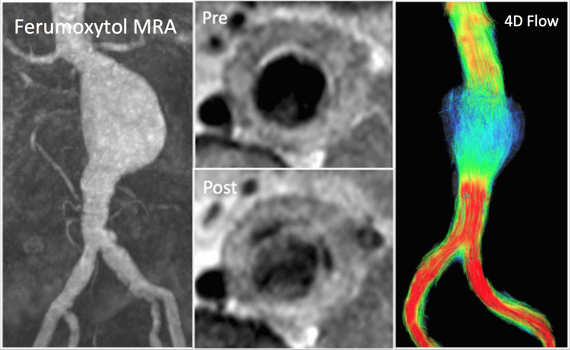
Goal: To elucidate the mechanisms of growth and rupture of small abdominal aortic aneurysms (AAAs) with a combined hemodynamic and inflammatory assessment using functional aortic imaging techniques.
Motivation: Abdominal aortic aneurysms (AAA) are common and can be life-threatening if they progress to rupture. They have been reported in 5% of older men and account for over 15,000 deaths per year. Basic vVessel dimensions are currently the primary imaging measurement clinically used clinically to risk-stratify patients. But there is more to the story than dimensions. Wall stress estimated with computational modeling may better predict growth and rupture than diameters. Growth is often not continuous, and instead marked by periods of rapid growth followed by quiescence. Small series report that unrelated surgical procedures can precipitate AAA rupture. These findings suggest that episodic and heterogeneous inflammatory processes in concert with adverse hemodynamics are important for the progression of AAA disease.
Rationale: The complexity of aortic disease is more fully revealed with new functional imaging techniques than with conventional anatomic analysis alone. While AAA has been extensively studied, the mechanisms of disease progression have not been fully elucidated. If better understood, the management of patients with small AAAs (< 5.5cm) could be significantly improved. Many of these aneurysms can be followed safely with a long screening interval of 2-3 years, but some may progress to rupture. Identifying this subset would greatly streamline the surveillance imaging of the millions of patients with AAA. On the other hand, the majority of AAAs never rupture, and identifying low risk patients could help better manage resources and subject only those patients at truly elevated risk to intervention.
We hypothesize that the systemic inflammation experienced with unrelated surgery will lead to AAA growth, and that this growth will occur at sites of unfavorable hemodynamics. Revealing such a combined inflammatory and hemodynamic mechanism for progression of AAA disease would constitute a substantial advancement in knowledge that would have both direct and broad clinical implications.
Strategy: Aortic wall inflammation can be evaluated with the MRI contrast agent ferumoxytol, which has macrophage-selective properties on delayed imaging. MRI also offers a unique and comprehensive assessment of aortic hemodynamics. Blood flow imaging with 3D time-resolved, 3D phase- contrast MRI (4D Flow) allows quantification of key secondary vascular parameters including turbulence and wall shear stress (WSS). Cine Displacement Encoding with Stimulated Echos (DENSE) can quantify regional stretch differences experienced by the vessel wall. Computational modeling based on MRI volumetric data can be used to calculate wall stress. We propose that analysis of these hemodynamic parameters along with transient inflammation will be central to understanding the progression of AAA disease., we have found an excellent and clinically relevant disease process for demonstrating the potential of 4D Flow in the management of patients with cardiovascular disease.
We intend to study patients with small AAAs before and after unrelated surgery with a combined inflammatory and hemodynamic assessment, and follow their progress with regular surveillance imaging.
Expected Outcome and Impact: The goal of our study is to uncover important inflammatory changes and adverse hemodynamics that are not addressed with current imaging, and use them to predict disease progression. Our approach is unique both in our targeting of patients undergoing unrelated surgery and our comprehensive use of new functional imaging techniques. We seek to meaningfully advance the assessment of risk in patients who do not meet current intervention thresholds and improve outcomes by refining surveillance imaging regimens and decisions regarding early intervention for AAAs.
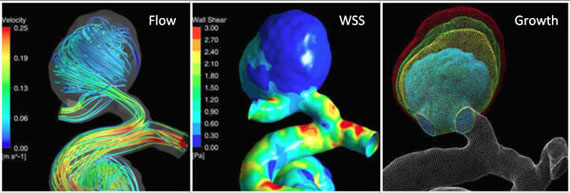
Intracranial Aneurysms
- 1R01NS059944-01A2 "Determinants of Intracranial Aneurysm Growth" (Saloner, 2009)
Accelerated 4D MRI
- 1K25EB014914-01A1 “4D MRI Development for Cardiovascular Imaging” (Liu, 2012)
Assessments of coronary artery disease and ventricular function are crucial elements for heart health evaluation. The goal of this research is to develop 4D magnetic resonance imaging (MRI) techniques that provide a non-invasive and effective cardiac imaging examination, including assessing coronary artery disease (CAD) and both left (LV) and right ventricular (RV) function.
Echocardiography (ECHO) is widely used to assess function but is limited to 2D views of the anatomy. It has poor ability to measure RV dimensions given the position, trabeculations, and complex anatomy of the RV. CT angiography (CTA) provides non-invasive assessment of CAD and ventricular function, however it involves exposure to ionizing radiation and risk of nephrotoxicity from iodinated contrast agents. Adequate CTA generally requires the use of beta blockers and is improved by the administration of nitroglycerin, both of which are contraindicated for certain patients. Coronary angiography (CA) is the definitive study for identifying the presence of CAD, but it is invasive, costly in both dollar terms and patient morbidity, and provides no assessment of ventricular function.
Cardiac magnetic resonance imaging (MRI) offers several powerful capabilities. It is the gold standard for quantitating ventricular function with 2D breath-hold cine acquisitions. Whole-heart coronary MRA centered on mid-diastole has also been demonstrated without contrast injection. However, both applications are limited by the challenges posed by cardiac and breathing motion. Cardiac function quantitation on 2D MRI suffers from breathing inconsistencies and consequent slice-to-slice spatial misregistration, particularly when patients, such as lung transplantation candidates, are unable to perform even a short breath-hold. Inconsistent breathing and selection of a fixed window at mid-diastole results in unacceptable scan times and reduces image quality in coronary studies since some coronary segments are not quiescent at that time.
In this research we aim to: 1) develop self-gated free-breathing 4D cardiac MRI with novel self-gating, motion correction, and advanced image reconstruction methods;2) optimize a 4D MRI protocol for cardiac function imaging and coronary artery imaging;and 3) implement the optimized methods in assessment of cardiac patients. Successful implementation of the proposed 4D cardiac MRI will not only be important for assessing CAD and ventricular function, but will be generalizable to conditions requiring similar capabilities, such as evaluation of valve disease, enhanced imaging of ischemic myocardium and assessment of vascular compliance. This will offer improved care for the vast population of patients with these conditions and financial benefits for the healthcare system.
People
Research Team
 Joseph Leach, MD, PhD
Joseph Leach, MD, PhD
Assistant Professor
[email protected]
 Jing Liu, PhD
Jing Liu, PhD
Professor
Data acquisition techniques; Image reconstruction algorithms; 4D MR imaging
 Dimitrios Mitsouras, PhD
Dimitrios Mitsouras, PhD
Associate Professor
[email protected]
 Yang Yang, PhD
Yang Yang, PhD
Associate Professor
 Huiming Dong, PhD
Huiming Dong, PhD
Postdoctoral Scholar
[email protected]
 Evan Kao, PhD
Evan Kao, PhD
Research Scientist
[email protected]
Computational Fluid Dynamics, Fluid-Structure Interaction, Data Visualization, Image Processing, Computational Geometry
 Jonas Schollenberger, PhD
Jonas Schollenberger, PhD
Postdoctoral Scholar
[email protected]

Alireza Sojoudi, PhD
Postdoctoral Scholar
[email protected]
 Keerthi Srivastav Valluru
Keerthi Srivastav Valluru
Research Engineer
[email protected]
 Yan, Wang PhD
Yan, Wang PhD
Postdoctoral Scholar
Medical image analysis, AAA segmentation, Aorta segmentation
 Ang Zhou, PhD
Ang Zhou, PhD
Postdoctoral Scholar
[email protected]
 Megan Ballweber, BS
Megan Ballweber, BS
Assistant Clinical Research Coordinator
[email protected]
Biomedical Imaging, Cardiovascular Disease
Collaborators
Diagnostic Radiology:
Neurology and Neurosurgery:
- Adib Abla, MD
- Christine Fox
- Heather Fullerton, MD
- Andy Josephson, MD
- Anthony Kim, MD
- Nerissa Ko, MD
- Wade Smith, MD, PhD
Cardiology, Vascular and Cardiovascular Surgery:
- Roselle Abraham, MD
- Warren Gasper, MD
- Liang Ge, PhD
- Edward Gerstenfeld, MD, MS
- Jade Hiramoto, MD
- Kendrick Shunk, MD, PhD
- Elaine Tseng, MD
- Yerem Yeghiazarians, MD
Neuro-Interventional Radiology:
- Matthew Amans, MD
- Daniel Cooke, MD
- Christopher Dowd, MD
- Steven Hetts, MD
- Randall Higashida, MD
- Kazim Narsinh, MD
Alumni
- Gabriel Acevedo-Bolton
- Loic Boussel
- Nicholas S. Burris
- Petter Dyverfeldt
- Farshia Faraji
- Henrik Haraldsson
- Cecilia Huang
- Daniel Hurwit
- Sarah Kefayati
- Andrew Lee
- Donne Nieuwoudt
- Vitaliy Rayz
- Monica Sigovan
- Sahand Sohrabi
- Bing Tan
- Mehrzad Tartibi
- Cheng Cheng Zhu
Vascular Imaging Research Center (VIRC) Directors
Related Content
- Research Directory
- Abdominal and Pelvic MRI
- Arthritis Imaging Lab (Li Lab)
- Baby Brain Research Group
- Biomagnetic Imaging Laboratory
- Biomechanics & Musculoskeletal Imaging Lab
- Bone Quality Research Lab
- BrainChange Study
- Breast Imaging Research Group
- Breast and Bone Density Group
- Cardiothoracic Imaging Imaging Research
- Center for Molecular and Functional Imaging (CMFI)
- Contrast Material and CT Translational Research Lab
- Evans Lab
- Focused Ultrasound Lab
- High Field MRI Center
- Imaging Research for Neurodevelopment
- Interventional Radiology Research Lab
- Larson Group
- Lupo Lab
- Multimodal Metabolic Brain Imaging
- Musculoskeletal Quantitative Imaging Research
- Neural Connectivity Lab
- Osteoid Osteomas HIFU Clinical Trial
- PET/SPECT Radiochemistry
- PSMA PET Scan
- Physics Research Laboratory
- Program for Molecular Imaging and Targeted Therapy
- Prostate Cancer Imaging Lab (Kurhanewicz)
- Quantitative Biomarkers & AI in MSK Imaging
- Research
- Sarah J. Nelson Lab
- Surbeck Laboratory
- Translational Metabolic Imaging Lab
- Vascular Imaging Research Center
- Viswanath Lab
- Wilson Lab
- Imaging Research Symposium
- Research Conference
- UCSF Radiology at RSNA
- Core Services

