Hyperpolarized Imaging Techniques for the Kidney, Brain, and Pancreas
Two studies from the UCSF Department of Radiology and Biomedical Imaging and the Hyperpolarized MRI Technology Resource Center (HMTRC) highlight new methods to better image cancer in the kidney, brain, and pancreas. Injectable hyperpolarized compounds enable imaging scientists using MRI to precisely measure the real-time metabolism rates of cells in those organs, identifying and mapping cancerous regions. This allows patients to receive more personalized and precise diagnosis and treatment monitoring of these cancers.
Measuring Transverse Relaxation Has Potential for Improving Kidney and Brain Imaging
"Dynamic T2* relaxometry of hyperpolarized [1-13C]pyruvate MRI in the human brain and kidneys" published in Magnetic Resonance in Medicine, discusses advanced imaging techniques for quickly and accurately measuring metabolism in the brain and kidneys. In addition to measuring metabolism, this new multi-echo imaging approach gives insight into the local tissue microenvironment via the transverse relaxation time (T2*). This provides an additional dimension of contrast, which can potentially be used to help stratify disease or better assess treatment response.
“Measuring the transverse relaxation is important for sequence optimization and can be used to improve the quantification of metabolism in HP 13C MRI,” said principal investigator Jeremy Gordon, PhD. “With a better understanding of these quantitative parameters, the acquisition design and accuracy of kinetic modeling in HP 13C MRI will be improved for future clinical studies.”
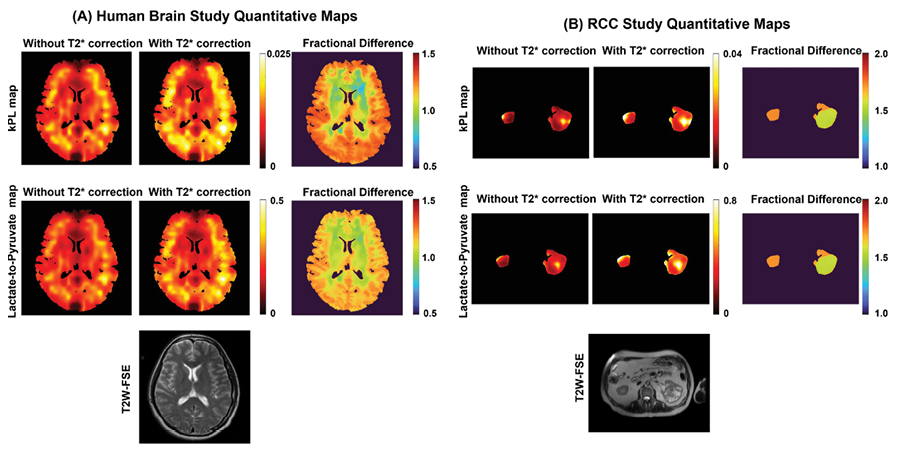
Though this study was primarily geared towards cancers of the brain and kidneys, the method could be useful in any disease in which normal organ metabolism is significantly altered. The researchers measured the transverse relaxation time (T2*) of hyperpolarized (HP) 13C-pyruvate and downstream metabolites in the brains of healthy volunteers and in the kidneys and tumors of renal cell cancer patients. They found that the T2* of pyruvate varied during the acquisition, while the T2* of lactate and bicarbonate were constant through time. The process also performed well in the kidneys without the need for prospective motion correction.
This new multi-echo dynamic imaging approach allows the quantification of both metabolism and the transverse relaxation (T2*) in a single integrated acquisition. Combining the data from all echoes can more than double the sensitivity of hard-to-detect metabolites such as bicarbonate, which can then be used to increase the spatial resolution to see finer details and better assess tumor heterogeneity. Gordon said, “We can also fit the multi-echo data to a signal model to measure the T2* of each metabolite. This parameter will be influenced by tissue microstructure and the local chemical environment and may provide further insight into disease aggressiveness or response to therapy.”
In addition to Gordon, the research team included Xiaoxi Liu, PhD, Di Cui, PhD, Duan Xu, PhD, Robert Bok, MD, PhD, Zhen Wang, MD, Daniel Vigneron, PhD, and Peder Larson, PhD.
Quantifying Changes in Pancreatic Tumor Metabolism May Indicate Early Therapeutic Response
Pancreatic Ductal Adenocarcinoma (PDA) is the third leading cause of cancer-related death in the United States, with a low survival rate and many challenges associated with both diagnosis and treatment. The current approach for assessing PDA treatment response relies on measuring changes in tumor size through computed tomography (CT) or magnetic resonance imaging (MRI) but this approach has many limitations.
Seeking a way to quantify metabolism in normal and PDA tissues and assessing changes in PDA metabolism following systemic chemotherapy, UCSF imaging recently published “Hyperpolarized 13 C Metabolic MRI of Patients with Pancreatic Ductal Adenocarcinoma” in the Journal of Magnetic Resonance Imaging (JMRI).
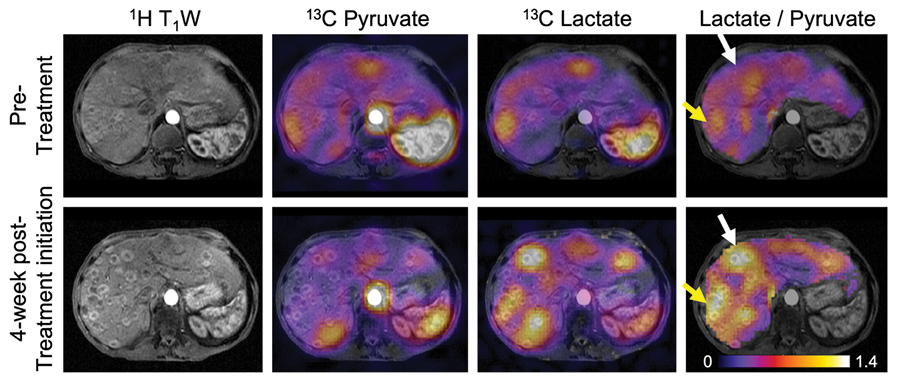
This study introduces a metabolic imaging technique that could enhance precision in assessing treatment response in patients with PDA. Six patients with locally advanced or metastatic PDA were selected for this prospective imaging study, and hyperpolarized [1-13C]pyruvate data were gathered before and four weeks after treatment initiation. By measuring pyruvate metabolism before and after the start of treatment, researchers can quantify changes in tumor metabolism that may be indicative of an early therapeutic response.
The study found HP 13C MRI to be especially effective at detecting changes in these pathways between normal appearing pancreas and PDA, or in the tumor following treatment. Hyperpolarized [1-13C]pyruvate is metabolized into lactate and alanine, both of which are products of pathways that are dysregulated in PDA.
Dr. Gordon said, “We observed an altered pyruvate metabolism in the primary tumor, characterized by reduced alanine-to-lactate ratios, and a correlation between early metabolic response and subsequent objective tumor response defined by response evaluation criteria in solid tumors (RECIST). These findings have potential clinical implications, as they highlight the role of metabolic reprogramming in PDA growth and therapy resistance and show the promise of hyperpolarized [1-13C]pyruvate MRI in assessing early treatment response.”
Metabolic reprogramming drives PDA growth and resistance to therapy. Because hyperpolarized [1-13C]pyruvate MRI can image pathways that are commonly altered in PDA, this imaging technology holds promise as a tool for improving the assessment of treatment response in patients with pancreatic cancers. Detecting an early metabolic response to treatment could enable more timely and accurate indicators of therapy response in PDA, allowing clinicians to promptly discontinue ineffective treatments and switch to alternative treatments with potentially better efficacy.
This study was authored by a collaboration of UCSF imaging faculty, both clinical and research, as well as trainees, and a research nurse. They are Jeremy Gordon, PhD, Hsin-Yu Chen, PhD, Tanner Nickeles, Philip Lee, Robert Bok, MD, PhD, Michael Ohliger, MD, PhD, Kimberly Okamoto, RN, Peder Larson, PhD, and Jane Wang, MD, with Andrew Ko, MD, from the UCSF Department of Medicine.
